News | Oct 14, 2020
Current AOP Developments: Curation of Mechanisms of Acute Systemic Toxicity
By Jessica Ponder, PhD, Regulatory Testing Analyst
Physicians Committee for Responsible Medicine
Posted August 7, 2020
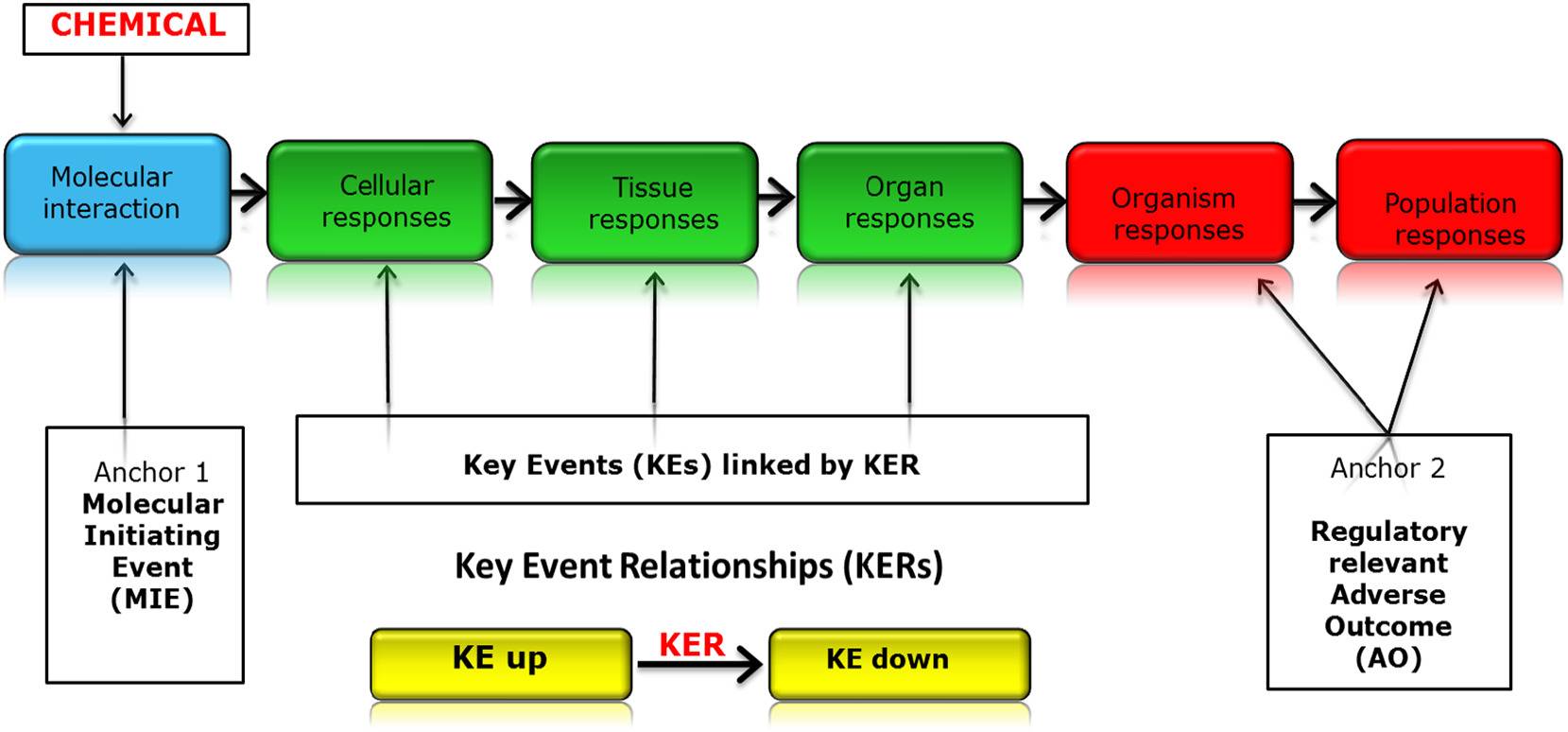
The OECD Adverse Outcome Pathway (AOP) framework is an innovative approach to organizing mechanistic knowledge of toxicological pathways, relying on collaborative sources to support the development of more effective and efficient chemical safety assessment methods. The AOPwiki is a public resource that features a modularly assembled database of mechanistic pathways leading to measured endpoints, traditionally those in clinical and animal studies. Given the quantity of biochemical pathways involved, crowd-sourcing can reduce workload and increase the volume of knowledge in the database by relying on users with the diversity of required technical expertise to contribute only small subsets of information into a modular format. However, without supervision, crowd-sourcing can lead to inefficiencies in the form of knowledge gaps, errors, and redundancies. For AOP development, more complex endpoints such as systemic toxicity are more sensitive to these drawbacks.
In order to maintain progress on the development of New Approach Methodologies for the Acute Systemic Toxicity endpoint, PCRM has established a partnership with an ICAPO Expert Group to perform a comprehensive cross-walk through the AOPwiki. The goal of this partnership will be to identify mechanistic pathways related to Acute Oral Toxicity according to recent literature reviews. A complete inventory will be generated to both organize the Key Events and Adverse Outcomes relevant to systemic toxicity and identify any gaps or needs for additional development of specific AOP components. Following completion, this curated inventory and gap analysis will be published as part of an open-access manuscript.
To read more about acute oral toxicity mechanisms, see Prieto et. al, 2019. For more information on this or other AOP developments, contact ICAPO@pcrm.org.
